News

December 8, 2025
March Biosciences Presents Positive Interim Clinical Data from Phase 2 Trial of MB-105 CD5 CAR-T Cell Therapy at ASH 2025 Annual Meeting
100% complete response rate achieved in primary cohort with acceptable safety profile in relapsed/refractory T-cell lymphoma patients

December 3, 2025
March Biosciences Announces Appointment of Gurpreet Ratra, Ph.D., as Chief Business Officer
March Biosciences welcomes Gurpreet Ratra, Ph.D., a biotech veteran with over 25 years of business development, strategy and operational experience, as Chief Business Officer
November 11, 2025
March Biosciences Receives FDA Regenerative Medicine Advanced Therapy (RMAT) Designation for MB-105 in Relapsed/Refractory CD5-Positive T-Cell Lymphoma
March announced that the U.S. Food and Drug Administration (FDA) has granted Regenerative Medicine Advanced Therapy (RMAT) designation to MB-105, the company’s CD5 CAR T cell therapy
All News Loaded
Publications
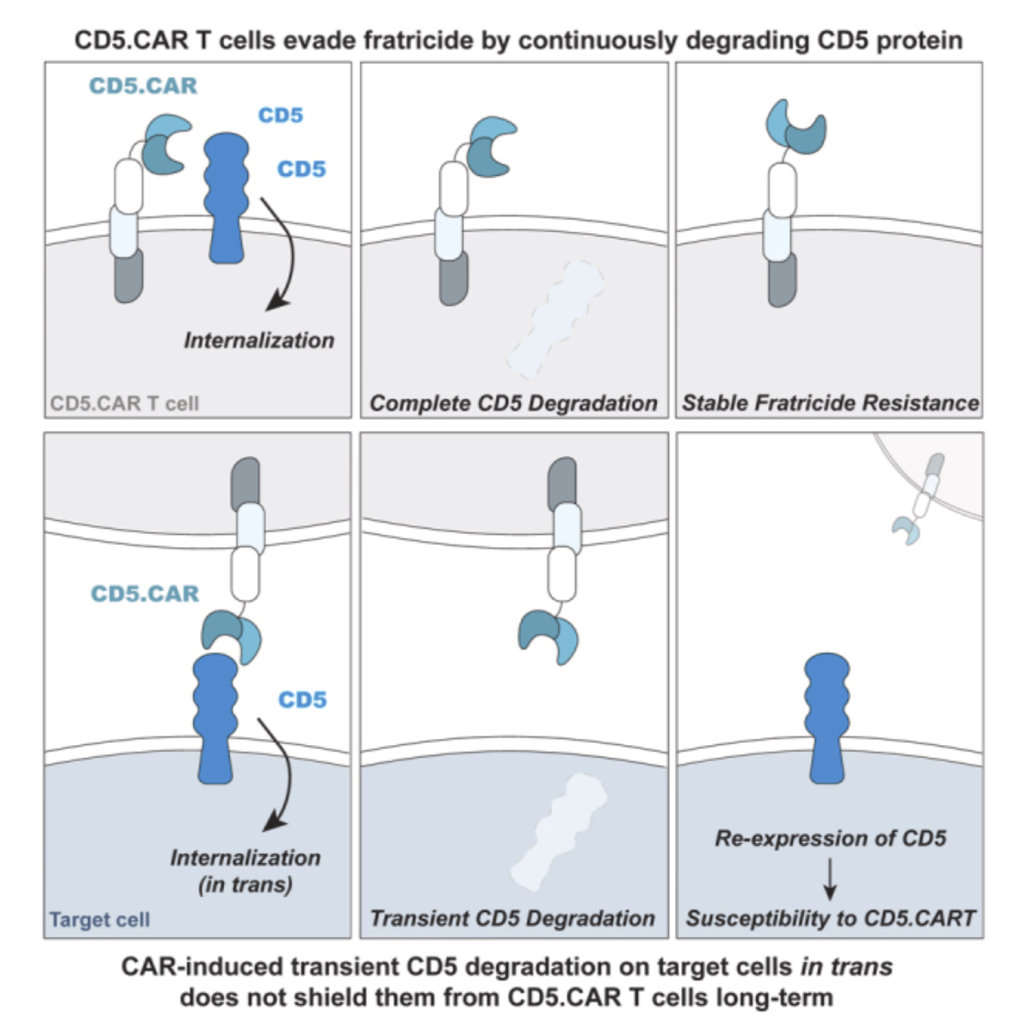
July 16, 2024
We demonstrate that the expression of a chimeric antigen receptor (CAR) targeting CD5, a prominent pan-T cell antigen, induces rapid internalization and complete loss of the CD5 protein on T cells, protecting them from self-targeting.

March 28, 2024
Phase 1 trial shows autologous CD5.CAR T cells are safe and effective in r/r T-cell lymphoma with a 44% overall response rate, promising advances in T-cell malignancy treatment.
June 2, 2023
We describe improved responses in T-ALL patients during a Phase I dose study (NCT03081910) of autologous CD5-directed chimeric antigen receptor T cell (CD5 CAR T) following the addition of tyrosine kinase inhibitors during manufacturing.